Product Categories
DO Meter Measurement Problems? | Check Out These Top Tips!
What is Affecting Your Dissolved Oxygen Measurements?
Dissolved oxygen in aquatic environments is critical to most species and understanding the DO levels in a system is equally important to aquatic managers, aquarists, researchers, and more. However, there are a few variables that can prevent you from acquiring accurate dissolved oxygen data. DO meter accuracy must take these 4 variables into account.
1) How Does Temperature Affect Dissolved Oxygen Measurements?
The most significant variable for dissolved oxygen measurements is - temperature. Making sure the temperature sensor on the instrument is accurately measuring is of the utmost importance as temperature has an impact on DO measurements in two ways.
Temperature and Diffusion
First, due to the increase or decrease in molecular activity, diffusion of oxygen through the membrane of an electrochemical probe or sensing element of an optical probe changes with temperature. The change in diffusion rate based on temperature can be up to approximately 4% per degree Celsius depending on the membrane material for steady-state electrochemical sensors, 1% per degree Celsius for Rapid Pulse sensors, and is approximately 1.5% per degree Celsius for optical sensors.
For example, if the temperature of a sample changes from 20°C to 15°C, the probe signal would decrease by varying rates depending on the sensor in use, giving a lower DO % saturation reading even though the % saturation of the water has not changed.
Therefore, the sensor signal must be compensated for changes in temperature. This is done by adding a thermistor to the circuit of older, analog instruments. For newer, digital instruments, the software compensates for temperature changes with proprietary algorithms that use the temperature readings from the probe’s thermistor.
Temperature and Oxygen Solubility
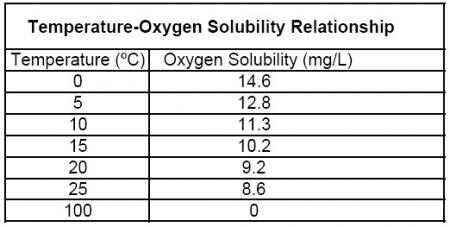
The adjustment described so far only compensates for temperature’s effect on the oxygen diffusion rate through a membrane or sensing element. In addition to this effect, temperature also affects the ability of water to dissolve oxygen. It is a scientific fact that the solubility of oxygen in water is directly proportional to temperature; see the Oxygen Solubility Table.
Colder water tends to dissolve oxygen more efficiently than warmer water. For example, in an oxygen-saturated sample of water at sea level (exposed to 760 mmHg of barometric pressure), the % saturation value will be 100% regardless of the temperature because it is fully saturated.
However, the dissolved oxygen mg/L concentration will change with temperature because the solubility of oxygen in water changes with temperature. For instance, at 15ºC water can dissolve 10.08 mg/L while 30ºC water can only dissolve 7.56 mg/L of oxygen even though the % saturation value is 100% in both samples. Therefore, we must compensate the mg/L concentration reading per the temperature of the sample.
Both of these temperature effects are factored into the conversion of the probe signal to an mg/L concentration. For newer, digital instruments such as the optical ProSolo and the traditional Pro20, the software compensates for both of these temperature-related factors after instrument calibration and during readings.
The temperature compensation for the % saturation reading is empirically derived, while the conversion from % saturation, temperature, and salinity to an mg/L concentration is automatically carried out by the instrument’s firmware using formulae available in Standard Methods for the Examination of Water and Wastewater. The calculation for converting % Saturation to mg/L and an example is provided below.
Determining Dissolved Oxygen mg/L from % Saturation
The following explains how to convert % Saturation to mg/L (also referred to as ppm or parts per million).
In order to perform this conversion, the temperature and salinity of the sample must be known. This is the reason accurate temperature values must be used in the calculation of mg/L values.
Step one: The percentage saturation, temperature and salinity of the given sample is what needs to be determined to begin with.
Step two: Multiply the % saturation reading by the value in the appropriate column (depends on salinity) and row (depends on temperature) of the Oxygen Solubility Table.
Example:
Step one: Sample is measured to have 80% DO saturation, 0 ppt salinity, at 20ºC
Step two: Multiply .80 (which is the DO %) by 9.09 (value from oxygen solubility table at 0 salinity and 20ºC) = 7.27 mg/L.
Result: 7.27 is the mg/L value that corresponds to an 80% DO Saturation reading of a sample with zero salinity at 20º C.
2) How Does Salinity Affect Dissolved Oxygen Measurements?
The second variable that affects DO concentration is the salinity of the water sample. While the % saturation reading is not a function of the salinity (or dissolved solids content) of the water, the mg/L concentration changes significantly with salinity.
The ability of the water to dissolve oxygen decreases as it’s salinity. For example, oxygen saturated freshwater with 0 ppt salinity at 25ºC contains 8.26 mg/L of oxygen while oxygen saturated seawater (~36 ppt) at the same pressure and temperature contains only 6.72 mg/L of dissolved oxygen.
Thus, salinity (along with temperature) must be factored into the instrument’s calculation of mg/L. This calculation is based on the % saturation reading, temperature reading, and the measured or entered salinity value using formulae found in Standard Methods for the Examination of Water and Wastewater. The calculation for converting % to mg/L and an example is provided above when we just reviewed the effects from temperature.
Correcting for Salinity
The salinity value used by the instrument in the calculation of mg/L is obtained in one of two ways, depending on the instrument being used. For YSI dissolved oxygen instruments that also measure conductivity (Pro2030), the salinity value measured by the conductivity sensor is used for the mg/L calculation. Therefore, it is important to ensure the conductivity sensor is calibrated and reading accurately in order to obtain accurate DO mg/L readings.
For YSI dissolved oxygen instruments that do not have a conductivity sensor, the salinity value of the sample must be manually entered by the end-user. See the salinity guide below for a list of typical salinity values for various types of water.
Salinity Guide - Average salinity by water type
|
Water Type |
Average Salinity |
|
Fresh Water |
<0.5 ppt* |
|
Brackish Water |
0.5 to 30 ppt |
|
Sea Water |
33 to 37 ppt |
|
Saline Water |
30 to 50 ppt |
|
Brine |
>50 ppt |
*Salinity is a unitless measurement determined from conductivity and temperature readings according to the Practical Salinity Scale which can be found in Standard Methods for the Examination of Water and Wastewater.
Historically, salinity values determined via the Practical Salinity Scale were given the designation “ppt” because these values were very close to those determined by the previously used method where the mass of dissolved salts in a given mass of water (parts per thousand) was reported. Today, ppt is commonly replaced by PSU (Practical Salinity Units) as the preferred unit to describe salinity calculated by the Practical Salinity Scale; however, these values are equivalent since they are determined by the same method.
When sampling water of varying salinity, for example in brackish waters such as estuaries or coastal wetlands, it is recommended that you use a dissolved oxygen instrument that also measures conductivity for the highest data accuracy. A dissolved oxygen instrument that also has a conductivity sensor will use the real-time salinity readings from the conductivity sensor for every mg/L calculation (such as the Pro2030, ProQuatro, ProDSS, or ProSolo ODO/CT). This will make sampling easier since it will not be necessary to manually change the correction factor at each sampling new site.
It's important to keep in mind when calibrating a DO meter, if you have to manually input the salinity value, to use the value of the water you will be measuring. If you have a conductivity sensor in conjunction with the DO sensor, make sure the conductivity is calibrated properly in order to compensate for the correct salinity value.
3) How Does Barometric Pressure Affect Dissolved Oxygen Measurements?
Another factor regarding potential effects in your dissolved oxygen calibration and measurement is barometric pressure.
Barometric pressure affects the pressure of oxygen in a sample of air or water. For example, the percentage of oxygen in the air is always 21%, but the actual pressure of oxygen varies with changes in barometric pressure. At sea level, the pressure of oxygen is 160 mmHg (.21 x 760 mmHg).
In a fully aerated sample, under these conditions, the % saturation measured by a sensor would be 100% (160/160 x 100%). If the temperature of the sample is 25ºC, the instrument would calculate the dissolved oxygen concentration as 8.26 mg/L based on the Oxygen Solubility Table. As the sample is moved up in altitude and kept air-saturated, the barometric pressure would decrease and so would the pressure of oxygen in the sample.
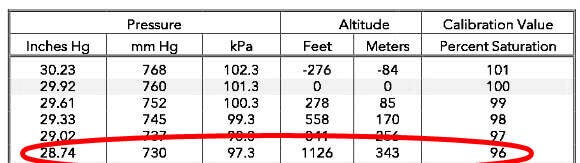
At 1126 ft of elevation, the pressure of oxygen would be 153 mmHg (.21 x 730 mmHg) and the % saturation relative to sea level read by the probe would be 95.6% (153/160 x 100%) in the fully aerated sample. If the temperature of the sample is 25ºC, the instrument would calculate a dissolved oxygen concentration of 7.92 mg/L or 96% of 8.26 based on the Oxygen Solubility Table.
The effect of barometric pressure is overcome by proper sensor calibration. Barometric pressure is used in the majority of dissolved oxygen sensor calibrations as described in the Calibration section of The Dissolved Oxygen Handbook since it determines the absolute pressure of oxygen in a sample of air or water at the time of calibration and it is this pressure that is measured by all oxygen sensors.
When calibrating oxygen sensors, the sensor’s output is set to this known pressure of oxygen. If the sensor output changes after calibration, then the instrument would calculate a % saturation based on a simple linear regression calculation.
Thus, as long as the system does not drift, the sensor’s output can always be used to define the oxygen pressure in any medium after performing a proper calibration and the use of the barometric pressure (or altitude) at the time of calibration is the key factor in setting the proper calibration coefficient.
Therefore, it is not necessary to correct for changes in barometric pressure after performing a proper calibration in order to obtain accurate readings in the field. OK, we're going to say this one again - seriously - we're repeating it and highlighting it.
It is not necessary to correct for changes in barometric pressure after performing a proper calibration in order to obtain accurate readings in the field.
In summary, as barometric pressure changes due to a change in altitude or local weather front, the pressure of oxygen changes. However, there is never any reason to compensate for this change if a proper calibration has already been performed and the sensor has not drifted.
As always, YSI recommends a proper daily calibration of your DO meter or, at the very least, a quick check to see if the DO % value is reading within +/-2% or +/-1% (depending on sensor technology) of what it should read at your altitude/barometric pressure.
Note: If DO% Local is being measured with a YSI 6-series sonde or 556 it may be necessary to recalibrate the instrument after extreme changes in barometric pressure or altitude in order to keep the DO% Local value at 100% in a fully saturated environment. This is not a requirement if only mg/L values are being recorded since these values will remain accurate without recalibrating Local DO %. If reporting DO% Local with newer instruments it is not necessary to recalibrate after a significant barometric pressure change in order to report an accurate DO% Local since these instruments have an on-board barometer that is read by the instrument continuously.
4) How Does Flow Affect Dissolved Oxygen Measurements?
The effects of temperature, Salinity, Barometric pressure are some of the many elements that can impact your dissolved oxygen meter measurements that we’ve given attention to. And now we will be moving onto flow dependence.
Steady-state electrochemical sensors, such as Clark (Dr. Leland Clark pictured) polarographic and galvanic, consume oxygen during measurement and therefore require sample movement, or the readings will be artificially low. This is commonly referred to as flow dependence since the sensor is dependent on the flow of water movement across the membrane in order to obtain accurate readings from the sampling environment.

Optical sensors (ProSolo, ProDSS, EXO), however, use a non-consumptive method for dissolved oxygen measurements resulting in a sensing method with zero flow dependence or stirring requirement.
However, YSI scientists have confirmed response times on optical sensors can be improved with flow. Accuracy doesn't change - just the amount of time to get to a reading which may be an advantage in the laboratory measuring many BODs or sampling DO in a lot of aquaculture ponds multiple times a day. A few seconds over many readings can add up in terms of time savings.
The graphs below illustrate this advantage of the optical sensor. The first is a graph of data measured with a steady-state polarographic sensor in an air-saturated water sample where adequate sample movement was provided by a mechanical stir bar. When the stirring mechanism was turned off, the readings began to fall resulting in artificially low dissolved oxygen measurements.
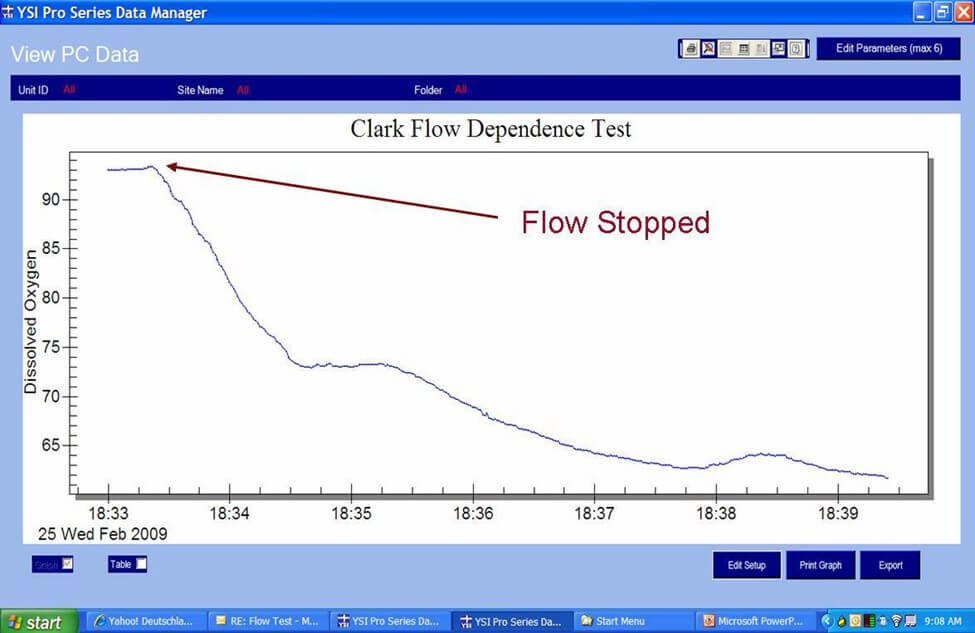
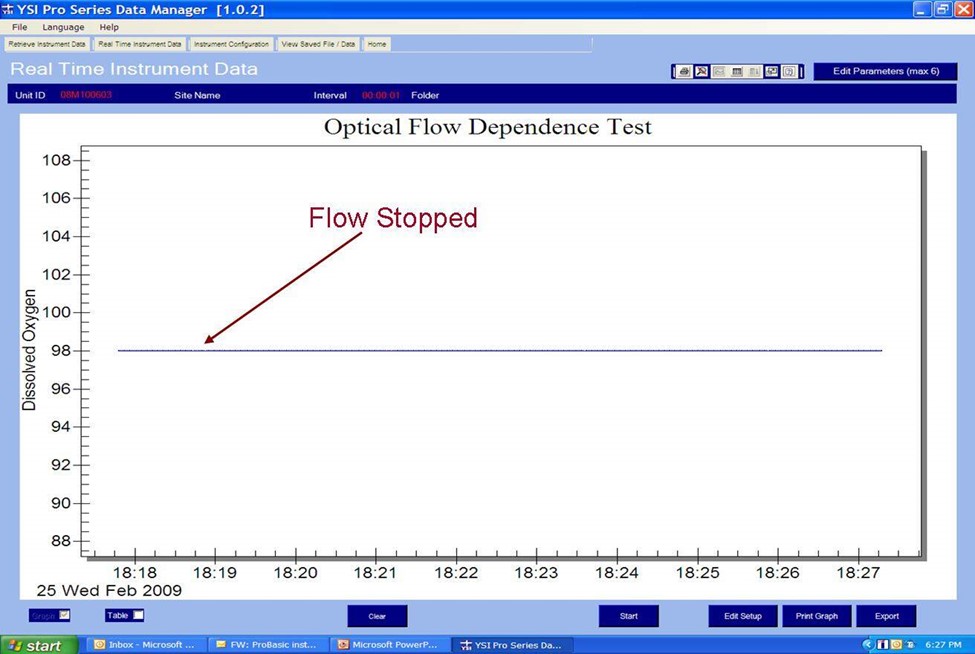
The second is a graph of data measured with an optical sensor in the same air-saturated water sample where sample movement was provided by a stir bar. When the stirring mechanism was turned off for the optical measurements, the readings remained constant and accurate proving the optical sensor is not dependent on flow. This is a considerable advantage of the optical sensor especially for low flow applications or applications where probe stirring or sample movement is difficult such as down well.
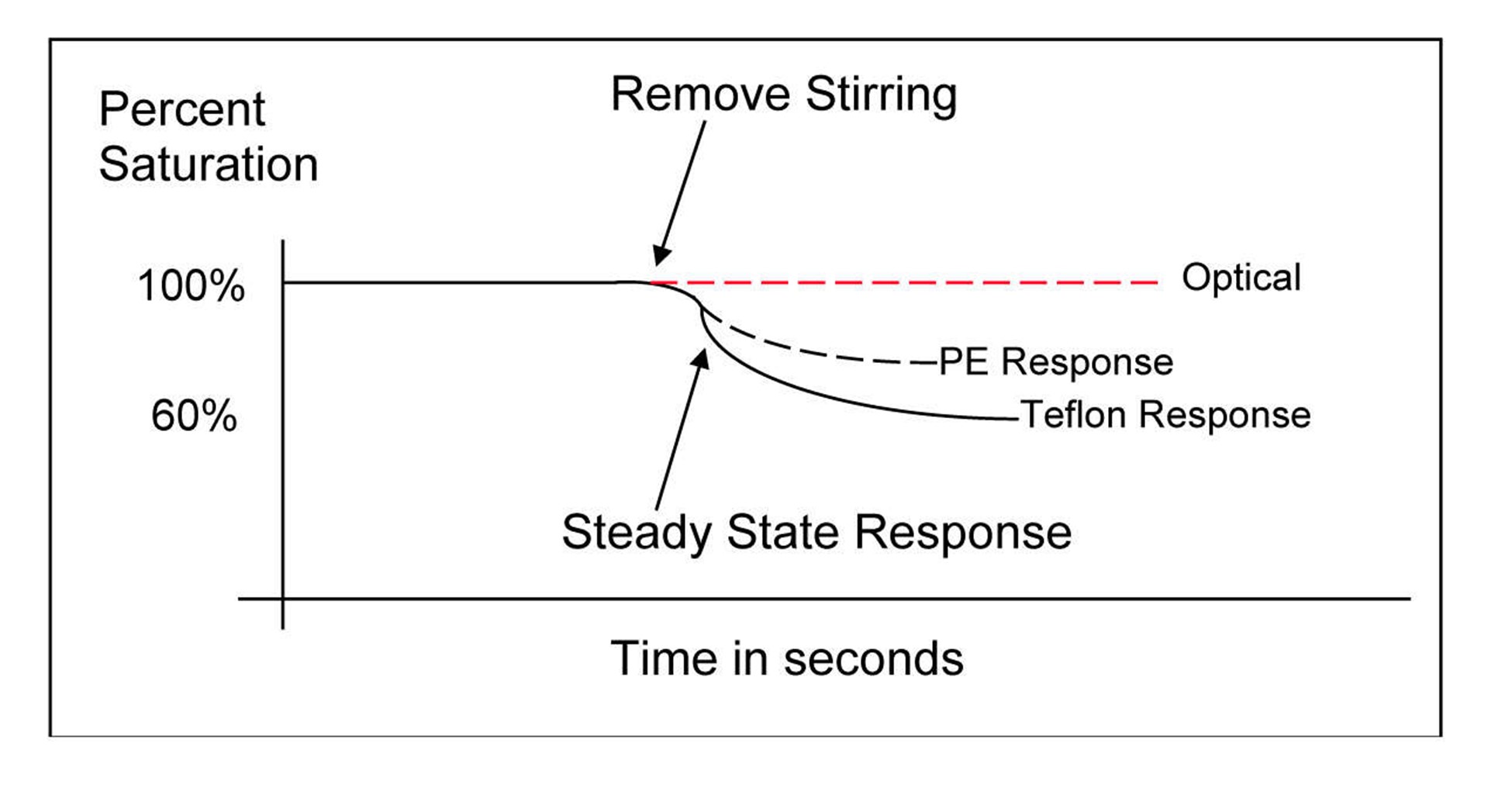
For steady-state electrochemical sensors, the membrane material and thickness dictate the degree of the sensor’s flow dependence. For example, polyethylene membranes, frequently notated as PE, require less movement or flow than traditional Polytetrafluoroethylene (you know it as a more common name starting with a T but we're not allowed to use it for legal reasons...) membranes as illustrated by the graph below.
In this graph, three different sensors were placed in fully aerated water with a stir bar. Once the stirring was ceased, the steady-state electrochemical readings began to fall. Notice how the Polytetrafluoroethylene-covered sensor fell further and more rapidly than the PE-covered sensor.
The stirring dependence of each sensor and membrane type, along with the recommended stirring rates, is listed in the Membrane Comparison Guide.
