
Flow Chemistry Basics & Key Elements
We’ve all heard of Flow Chemistry, especially at a time when everyone from chemistry professors and students, to research and development chemists are working, studying and perfecting flow chemistry.But do you know what flow chemistry actually is?
Learn from Syrris’s expert Dr. Andrew Mansfield about what flow chemistry is, how it works, and the different types of mixing junctions available.
Some of the Highlights:
The Basics of Flow ChemistryWhat is the difference between Plug Flow & Continuous Flow Chemistry?
Why are Chemists Working with Flow Chemistry?
Key Element: Mixing Junction & Flow Reactors
The Basics of Flow Chemistry
Flow Chemistry may have many names: “plug flow chemistry”, “microchemistry”, and “continuous flow chemistry” but the principles of flow chemistry, or the flow chemistry basics, are the same: the process of performing chemical reactions in a tube, capillary or micro structured device (a flow reactor). Continue reading further to learn more about Flow Chemistry process and key elements.
Dr. Mansfield explains further, “what this means is that reactive components are pumped first through a mixing device, either a t-junction or a static mixer and then flowed down a temperature-controlled flow reactor; a radically different approach from the traditional chemistry method of performing reactions in glass flasks or jacketed reactors. Further to maintaining a fixed temperature to promote a reaction flow chemistry lends itself perfectly to using photon or electron flux to mediate continuous reactions.”
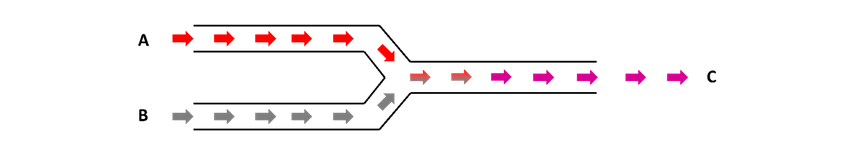
What is the difference between Plug Flow & Continuous Flow Chemistry?
Continuous Flow Chemistry is explained in the name: continuous. In continuous flow chemistry you get a continuous stream of end product, as the reactive materials are pumped with no breaks, creating continuous a stream of chemicals.
Whereas in Plug Chemistry, alternating “plugs” of reactive materials and solvent are pumped, where each plug is considered as a separate entity (a discrete reaction mixture).
These plugs never meet – they are separated by the system solvent – so the conditions in which they go through the flow chemistry system (i.e. temperature, stoichiometry, residence time etc.) can be changed to observe how the reaction changes. This type of flow chemistry is ideal for method development, reaction optimisation, and library synthesis, as smaller amounts of material are required.
Syrris’s intelligent systems like the Asia, are able to automate these processes, which enables a range of reaction conditions, reagents or analogs to be explored with the automated collection of individual plugs, sending the product into one collection vial and the solvent to waste.
Why are Chemists Working with Flow Chemistry?
Precise Control. With Flow Chemistry one can control several reaction conditions such as stoichiometry, mixing, temperature control, and reaction time. Having control over these factors allows for excellent reaction control often leading to greater yields and better selectivity.
There are many reasons chemists across all industries are introducing or switching to, continuous flow chemistry, however Dr. Mansfield highlights the main ones:
- Faster reactions
- Safer reactions
- Faster reaction optimization
- Fast serial library synthesis
- Reaction conditions not possible in batch
- Reactions are usually more selective
- Scale up is easier in flow than batch
- Easy integration of reaction analysis
- Reactions are easier to work-up in flow
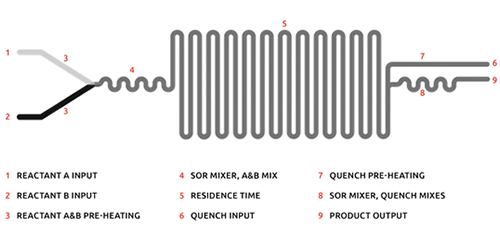
Key Element: Mixing Junction
A mixing junction is where the reagent streams meet before flowing into the flow reactor. Usually, a simple t-piece will do, however if you need faster and more efficient mixing, a static mixer can be used. The two types of mixing junctions contribute to different mixing regimes, diffusive and turbulent. For reactions that require fast mass transfer, turbulent flow, using a micromixer (static mixer) is necessary.
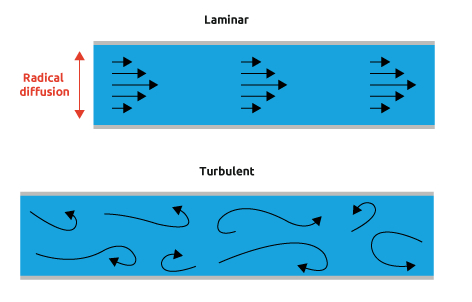 Dr. Mansfield explains that, “when a fluid is flowing through a closed channel such as a flow reactor, either of two types of flow may occur depending on the velocity and viscosity of the fluid: laminar flow or turbulent flow. Laminar flow tends to occur at lower velocities, below a threshold at which it becomes turbulent. Turbulent flow is a less orderly flow regime that is characterized by eddies or small packets of fluid particles, which result in lateral mixing. In non-scientific terms, laminar flow is smooth, while turbulent flow is rough.”
Dr. Mansfield explains that, “when a fluid is flowing through a closed channel such as a flow reactor, either of two types of flow may occur depending on the velocity and viscosity of the fluid: laminar flow or turbulent flow. Laminar flow tends to occur at lower velocities, below a threshold at which it becomes turbulent. Turbulent flow is a less orderly flow regime that is characterized by eddies or small packets of fluid particles, which result in lateral mixing. In non-scientific terms, laminar flow is smooth, while turbulent flow is rough.”When mixing in laboratory scale flow systems, like the Asia, mixing will occur through the lateral mixing regime of laminar flow. Although diffusion mixing can be slow, it is very significant and more reproducible.
Key Element: Flow Reactors
A jacketed reactor or round-bottom flask where chemical reactions take place continuously. There are many shapes and sizes available, however, Dr. Mansfield pinpoints a few features one should look for when choosing a flow reactor:
- Flexibility in volume to allow a large range of residence (reaction) times
- Excellent mixing
- Excellent heat transfer
- Good visibility where possible
- Ability to perform different types of chemistry (i.e. Homogeneous and heterogeneous)
 The following types of flow reactor are all available with the Syrris Asia Flow Chemistry System:
The following types of flow reactor are all available with the Syrris Asia Flow Chemistry System:Glass Microreactor Chips
Most common type of reactor used in flow chemistry. The design of the glass microreactor chip depends on your application – it helps determine how wide the mixing channel is and how the mixing occurs. The longer the channel, the longer residence time, assuming the pump flow rate is the same.
Glass microreactor chips are inserted into chip climate controllers which allows to maintain a set temperature throughout the entire chip. Dr. Mansfield advises that these are the perfect system for chemists just starting out in flow chemistry.
Tube Reactors
Tube reactors are long tubes wrapped around a heated or cooled coil (the large length of the coil offers better residence times than glass microreactor chips, or much faster pump flow rate). Available in different materials, for different applications. PTFE offer good visibility but temperature restrictive. For high-temperature and pressure reactions stainless steel and Hastelloy are better.
Column Reactors
Typically glass columns that allow heterogeneous chemistry to be performed. Solid-supported reagents, catalysts, enzymes, and scavengers can be used with columns reactors as passing solids through small diameter tubes isn’t always easy.
You can find the full article from Dr. Mansfield here.
Want to Know More?
If you are still using traditional chemistry techniques, do investigate flow chemistry. Contact us today if you want to discuss your chemistry needs. Our Team will be happy to help.
Contact Us Today! |
