Product Categories
How to Achieve More Accurate Titrations
Titration is a well-known method of determining the presence of an analyte in a sample. The techniques have remained in use due to their simplicity and general correctness. But don't be fooled: when it comes to tactics and practices, there's always space for growth. There is a lot of variation in test findings from analyst to analyst, and there are a lot of things that might impact how accurate your results are.
Improve the Accuracy of Titrations in Your Lab
Standardize Your Titrant
“When is the last time you’ve standardized your titrant?” When troubleshooting or questioning a result, this is the first thing we ask our consumers.
The technique of standardization normalizes the titration system and gives the most precise titrant concentration. This number is crucial in determining the analyte content at the end. It can throw off a result if the concentration isn't determined exactly. Some titrants, for example sodium hydroxide, can absorb CO2 from the air causing the true concentration to decrease. You can minimize the atmospheric influence on these hygroscopic titrants by using a CO2 absorption tube and filling it with soda lime. However, the best practice is to perform titrant standardization.
Titrant standardization is typically performed in triplicate at a minimum. A % RSD (Relative Standard Deviation) is calculated and a value of 1% or less is accepted in most GLP (Good Laboratory Practice) laboratories. The YSI titrators are pre-programmed with standardization methods for the most common titrants (ie NaOH, HCl, H2SO4, AgNO3, and many others) which make it simple to implement this procedure into practice.
Image 1 (below) illustrates an example of a common standardization procedure on a strong base.
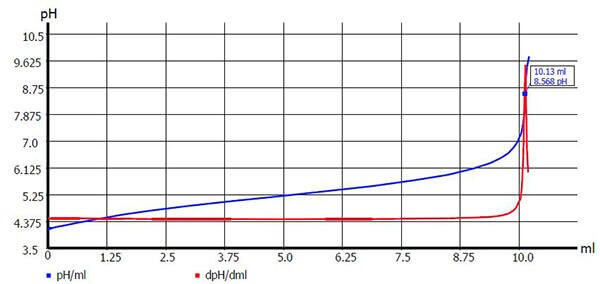
Image 1 : Titer determination of 0.1 mol/l NaOH with potassium hydrogen phthalate
Standardization should be done whenever new titrant is put to the reservoir bottle or on a regular basis (monthly or bimonthly).This titrant concentration check is an excellent place to start if you have any doubts about the findings (high or low).
Homogenize Your Sample

Homogenizing might be a good idea depending on the type of sample you're testing. Homogenization is a mechanical procedure that ensures that everything is the same. When it comes to titration, homogenization might make the analyte molecules more "accessible," allowing them to react with the titrant.
The titration for salt levels in cheese is a good illustration of this. With merely a magnetic stir bar and stir plate, it's tough to break down the sample and release the salt. A homogenizer can help break down the sample so that the salt can be released, and the titrant can react completely. Without a homogenizer, you may see lower results than expected. If you need help choosing a homogenizer, let us know!
Check Your Sample Measuring Techniques
For you, fellow chemists, we may be taking a journey down memory lane. Remember when you learnt how to use class A volumetric pipettes in general chemistry classes? Or how essential weighing techniques were? It is critical that you measure your sample precisely, regardless of how you do it. It's easy to see why when you look at the basic math used in titrations:
C2 = C1 * V1
V2
C1 = Concentration of titrant
V1 = Volume of titrant consumed at end point
C2 = Concentration of analyte in sample (UNKNOWN)
V2 = Volume (or weight) of sample
The volume of the sample is utilized to calculate the amount of analyte in the sample in the above computation. Any inconsistencies in this V2 value will have an impact on your outcomes. Glassware accuracy is often quantified in terms of tolerance when measuring liquid samples.

This tolerance refers to the degree of uncertainty in the measurement done using glassware. Graduated cylinders have a volume measurement error of 0.5 to 1 percent. In comparison to graduated cylinders, volumetric pipettes often provide the appropriate volume within 0.1 percent. Because sample quantities in titration studies are often modest (often less than 20ml), working using volumetric pipettes is a good option.
A calibrated balance should be used to weigh solid materials. The sort of balance used will be determined by the required number of significant figures in the result. A two-place balance, for example, would sufficient if the result had to be shown to the hundredths place. A four-place balance, on the other hand, should be utilized if your findings are published to the fourth decimal. For the most precise weight, use a calibrated and steady analytical balance.
Calibrate Your Electrode Regularly
This one may seem self-evident, but it's worth repeating. If you're doing a titration with an electrode, you should be aware that the probe's condition determines whether the measurement is successful or not.
To achieve the best accurate result, a pH electrode should be calibrated every day (at the very least once). If the proper buffers are used, a two-point approach is usually sufficient. What exactly does "suitable" imply? Buffers should be selected based on the measurement range that you use on a regular basis.
For example, if your titrations typically take place in the 5-6 pH range, you should select pH buffers 4 and 7. Using fresh and clean buffers is also important for a successful calibration.
The reaction time of an electrode will grow over time, indicating that calibration should be done more often. The offset and slope may also alter, dictating how frequently the calibration should be performed.
The following are commonly used limitations within which an electrode is still regarded reliable:
- Slope 95% to 102%
- Zero-point pH 6.5 to pH 7.2
A well-preserved electrode with a good calibration can last an average of 1.5-2 years depending on the frequency of use. If you have any questions or need assistance choosing the right electrode for your application just ask our Sales Team or learn more about pH.
Check for Air Bubbles in the Burette
Air bubbles can occur in your burette whether you're manually titrating or using an auto titrator. Because air takes up space in the burette and the measured amount of titrant is more than what is really ingested, this might lead to falsely low readings.
Air bubbles can develop at the tip of the stopcock burette in manual stopcock burettes. Using the burette and/or purging several millilitres of titrant through it, they can be eliminated. Air bubbles can develop in the burette cylinder or in the titration lines when using an autotitrator or a piston burette. Any air bubbles will be removed by flushing the titrant through the burette and tube.
To Find Out More |
|
