What is Flow Chemistry?
Flow Chemistry is the process of performing chemical reactions in a continuous pipe or tube. This chemical process is also known as continuous flow chemistry or plug flow microchemistry.
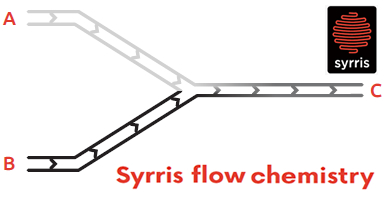
Reactive components are pumped together at a mixing junction and flowed down a temperature-controlled pipe or tube. This provides some major advantages, such as faster and safer chemical reactions, cleaner products, and also easy scale-up.
How Does Flow Chemistry Work?
The experts at Syrris, lead company in flow chemistry and batch chemistry reactor systems further explain that “the ratio of the reactants is controlled by their concentrations and relative flow rates. The rapid mixing and large surface area to volume ratio provides excellent reproducibility and control of a chemical process.”
What Are the Benefits of Flow Chemistry?
Faster Chemical Reactions
Flow reactors are easily pressurised (e.g. Asia systems can be pressurised to 300psi). This allows reactions to be heated 100-150ºC above their normal boiling point, therefore, creating reaction rates that are 1000s of times faster. This process is called superheating.
Cleaner Products
Flow reactors enable excellent reaction selectivity. The rapid diffusion mixing avoids the issues found in batch reactors. The high surface area to volume ratio (1000x greater than a batch reactor) enables almost instantaneous heating or cooling and therefore ultimate temperature control.
Safer Chemical Reactions
Flow chemistry allows only a small amount of hazardous intermediate to be formed at any instant. The high surface area also allows excellent control of exotherms.

Integrated Synthesis, Work-Up and Analysis
Reaction products exiting a flow reactor can be flowed into a flow aqueous workup system or solid phase scavenger column. From there they can be analysed either in line (e.g. FTIR) or a sample taken, using a sampler and diluter then and injected onto and LCMS.
Rapid Reaction Optimisation
Flow Chemistry with automation enables the quick variation of reaction conditions on a very small scale e.g. 100 µL. Parameters such as reaction time, temperature, ratio of reagents, concentration and reagents themselves can all be rapidly varied. One reaction can follow another, separated by solvent, each cleaning out the previous reaction.
Easy Scale-Up
Scale-up issues are minimised due to maintaining excellent mixing and heat transfer. Higher flow rates and correspondingly larger reactors can be used to easily produce kilogram quantities.
Reaction Conditions not Possible Using Traditional Batch Chemistry Methods
Flow chemistry facilitates reaction conditions not possible in batch such as a 5-second reaction at 250 ºC. Multi-step procedures such as a rapid low-temperature deprotonation followed instantaneously by the addition of an electrophile high temperature are made easy.
Flow Chemistry Reaction Examples
Below is an application note showcasing a chemical reaction example using Syrris’ flow chemistry systems. Syrris’ innovative microreactor-based systems include the modular Asia and Titan.

Oxidation of a Primary Alcohol
This paper describes reaction conditions for the oxidation of alcohols in continuous flow using a column reactor packed with polymer-supported tetra-N-alkylammonium perruthenate.
- Chemistry: Organic Synthesis
- Authors: Steven V. Ley, Ian R. Baxendale, Jon Deeley, Charlotte M. Griffiths-Jones, Steen Saaby, Geoffrey K. Tranmer (Cambridge University)
- System: Syrris Asia Flow Chemistry
- Title: A Flow Process for the Multi-Step Synthesis of the Alkaloid Natural Product Oxomaritidine: A New Paradigm for Molecular Assembly
Download the White Paper! |
Want to Know More?
If you are still using traditional chemistry techniques, do investigate flow chemistry. Contact us today if you want to discuss your chemistry needs. Our Team will be happy to help.
Contact Us Today! |
